
5-day Review of 510(k) Submission
Please read our Terms and Conditions before purchasing.
Alternatively, Contact Us if you need more information! We would be happy to speak with you about your specific needs.




Please read our Terms and Conditions before purchasing.
Alternatively, Contact Us if you need more information! We would be happy to speak with you about your specific needs.

Please read our Terms and Conditions before purchasing.
Alternatively, Contact Us if you need more information! We would be happy to speak with you about your specific needs.

This is a change control standard operating procedure bundle. It includes a change control SOP template along with all supporting documents such as: change request template, action plan template, and verification results template. We designed it for medical device companies that want to fast track documenting their change control procedure. It is compatible with ISO 13485, the EU Medical Device Regulations 2017/745 and/or the FDA 21 CFR 820.

Documents in a medical device file may require duplicate information. For example, the intended use is a statement that appears in many documents such as the device description, project plan, risk management plan, or sterilization validation, etc. Modularizing the documents may improve your documentation quality by avoiding copy paste errors, but may also reduce the time needed to copy and paste as well as review burden.

Need to check the content of a standard before purchasing, so that you know whether or not it applies to your product? Or perhaps you want to clarify a reference to a standard in a document sent to you by one of your suppliers? You can access the many standards for free!
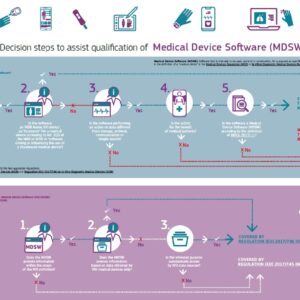
Is the software you’re developing a medical device?
This infographic published by the European Commission may help you figure it out.
