Power your medical device to market!

Get it all
One platform for all business activities
Launch any business activity from one location and simply follow the prompts.
With Conformify you can:
- Deal with just the business activities relevant to your role
- Customize business activities to meet your organisational needs
- Cover all your regulatory and quality business activity obligations in one place
- Cover business activities that are not quality or regulatory related
Build compliant projects of any complexity with ease
Have a development project with hardware, software, electronics, biologics? Another with just hardware?
Conformify processes are modular so you only add what you need.
Everything you need in one place
Don’t waste time assembling everything you need to complete a task. Conformify does that hard work for you.
- Get background and supporting information
- Get related documentation
- Get needed templates, checklists, forms etc.
- Upload the deliverable documentation
Features
Complete QMS
Project Management
Resource Management
Keep up-to-date with progress.
Technical File Management
Audit Management
Ensure coverage of standards or regulations. See how they are covered by business processes. Easily identify gaps.
Library Management
Quickly find and view all processes including their related templates, forms or checklists. See all related documentation.
Training Management
Compatibility
Fully Customizable
Frequently Asked Questions
To understand the magic of Conformify, it might be best to work through an example.
Imagine you are an organisation that has a medical device cleared for a specific market and you want to make a change to it. Let’s step through how Conformify will support you from A to Z.
Step 1. In the Conformify software, you should launch a new business activity by scrolling down to select ‘I want to make a change to an existing approved device’. If you are not allocated the rights to make changes to devices, you will not be able to launch this business activity.
Step 2. Next, you must work through the wizard to build a project. For example, this wizard explains that you first need to have an approved change request and action plan before making changes to an approved device using the change management process. So, you need add a change management work package to the project.
Step 3. The work package requires a change requestor (one that is responsible for filling out details about the change and coming up with an action plan) and change request reviewers (those that can approve the change). Conformify automatically detects that these resources are required and prompts you to associate employees that have these roles to the project. You can also see whether the employee has completed the necessary training. You can still add them to the project if the training is not complete, but they will not be able to complete any tasks until this training is undertaken.
Step 4. Now the work package and resources are added, Conformify automatically creates the first task of the change management work package and assigns it to the correct resource. In this case, a task is created for the change requester to fill out a change request for approval that describes the change that is required and an assessment of the impact of the change.
Step 5. The change requestor receives this new task in their inbox. By clicking on the task, they get a complete explanation of what is required of them directly from the SOP (to fill out the change request) and are automatically presented with the relevant change request form for them to download and fill out.
Step 6. The change requestor fills out the form and uploads it to the task and presses complete.
Step 7. A task is automatically created for the change reviewers
Step 8. The change reviewers open the task and can read exactly what they need to do (direction from the SOP). They are also presented with the change request form that was previously filled out. They can then download and review this form. Once they have reviewed it, if they have any comments, they can download and fill out the presented comments form and upload this and press complete. They then can select that the change request needs an update, so a new task is created for the change requestor and the comments are then presented to them.
Skipping through several steps, let’s see how it is when adding documents that form part of the technical file.
Step 23. Now a task has been allocated to the project requirements engineer to create requirements for the software of the device. The requirements engineer downloads the User Needs and System Requirements for review and then downloads the software requirements template and fills it out. They then upload the document and associate the document with the software component and the version to which they apply.
Step 24. The project manager wants to see how the technical file is coming together and goes to the device version that is currently under change. As the software is a component of the device, the requirements are also associated with the device so the project manager can see this in the technical file.
Skipping through to the end of the project…
Step 62. Go to the device documentation dashboard and filter for ‘FDA 510(k) file’ and see all the documents that should be compiled together for a 510(k) submission. Select the documents that you need for your ‘update 510(k)’ submission.
Not just a data manager
Most QMS software out there is designed to manage your quality data. This means that it can maybe trace your requirements to your risks and test cases, or provide you with customer complaint forms and workflow, but you still need to create your own Standard Operating Procedures (SOPs), templates, etc. to be compliant.
Most QMS software only really deals with ISO 13485 clauses of the quality system and does not support other aspects such as developing a clinical evaluation, reporting to authorities, post market surveillance requirements, etc. Conformify supports all these and more.
Has everything you need
When going into the market with a medical device, you don’t just need to worry about a QMS. You also need to worry about other regulations that you are subject to, such as periodic reporting, post-market surveillance which could include post-market clinical trials. Conformify can support all of this.
Fully customizable
Other QMS software promises to be customizable, but in the end you can only really change some forms or some minor aspects of workflows. This may not be enough for you. Also, they may charge you excessive amounts to make this customization.
Works out of the box
Many QMS software requires a large amount of configuration before you can even start with the software, which can be expensive and time consuming. Conformify works out of the box. Simply upload the SOPs and documents you need, and you are off!
Doesn’t require excessive training
Many QMS software on the market have a steep learning curve and so these companies provides days of expensive training before you can get up and running. Conformify requires a small amount of training and then the rest is self-explanatory. The wizards take care of the rest.
We can provide quality management system documentation, including SOPs, templates, checklists, forms, etc. for any aspects of the quality management system you require. You do not need to buy a whole ‘module’, you can even pick and choose at the process level what you actually need.
We can also support processes and documentation not under a QMS, for example if you have a special way of ordering stationary for the organisation, we can support that too.
You can mix and match the exact processes that you need. Using a separate piece of software to manage your customer complaints? Then you don’t need any of our customer complaints processes or documents. Already implemented a production process? Then you don’t need our production processes and documents.
Unlike other eQMS software, you can customize exactly what you need very easily. Our software is designed to read and navigate the processes that are fed to it. All you have to do is send us your SOPs, documents, etc. and we will convert them into a Conformify format.
It’s that simple.
Conformify software reads and interprets processes that are fed to it, so the software itself has no pre-programmed quality management system. That means that you can use our processes either with the software or as a standalone paper based quality management system if you like. It is also how we can so easily accommodate any customization you may wish.
Conformify is a cloud based software built on Microsoft Azure using state-of-the-art security practices.
You will also have geo-redundant storage of your data and where possible we will provide that within your own country so it never leaves your jurisdiction.
Conformify can support you to update processes and documents where necessary. Or, you can send us what you want updated and we can convert it to a Conformify format for you. All license models include this support for free.
Latest Blogs

Make a killer 510(k) – Part 2
One option to get more information about what the FDA may be looking for is to check the 510k database for similar devices that have been subjected to a Freedom of Information request, or FOIA Request.

Make a killer 510(k) – Part 1
Looking at the classification order or the reclassification order for device types can give you a lot of insight into the FDA’s thoughts on the benefit risk argument for your device. Addressing the FDA identified risks using the special controls identified for each risk correctly can boost your 510k clearance chances.
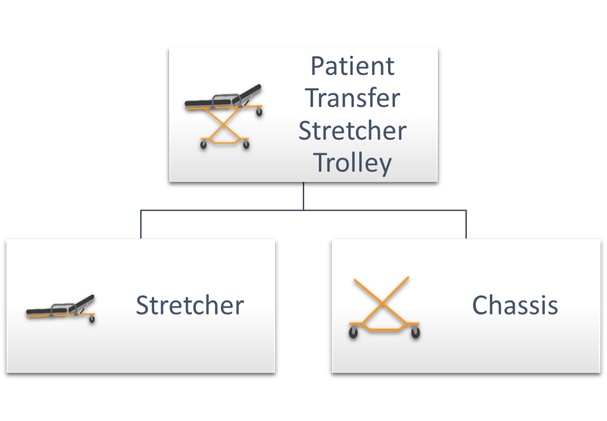
User Needs to Testing: A Complete Example – Part 3: Requirements
We’ve all seen the tree swing analogy. Each member of a project has a different idea about the final product and it results in several misunderstandings.
How could this have been avoided? With good requirements!
Requirements are a contract between the business, the user and the design team. It transforms the user and business needs into a language that guides design, but does not dictate it. It captures all the goals that the design needs to meet in easy to understand language.


