Blog
Tags
Categories
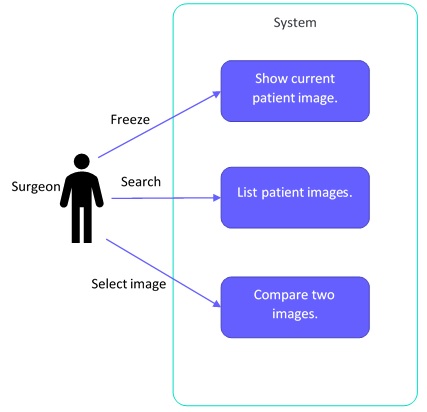
User Needs to Testing: A Complete Example – Part 2: User and Business Needs
So, what are ‘User and Business Needs’ and why do we need them?
Medical devices, at a fundamental level, need to be safe and effective. More specifically, they need to be safe and effective when used in the context of their intended use. Regulatory markets around the world have recognized that ensuring that manufacturers consider users throughout design and development is critical to the success of a device and the safety of patients.
We take you through how to create a high quality user needs document with some examples.

ISO versus EN ISO
Ever wondered whether it was sufficient to use ISO 14971 instead of EN ISO 14971 for the European medical device market? This gives a quick breakdown of what the difference is, and why you need the EN ISO.
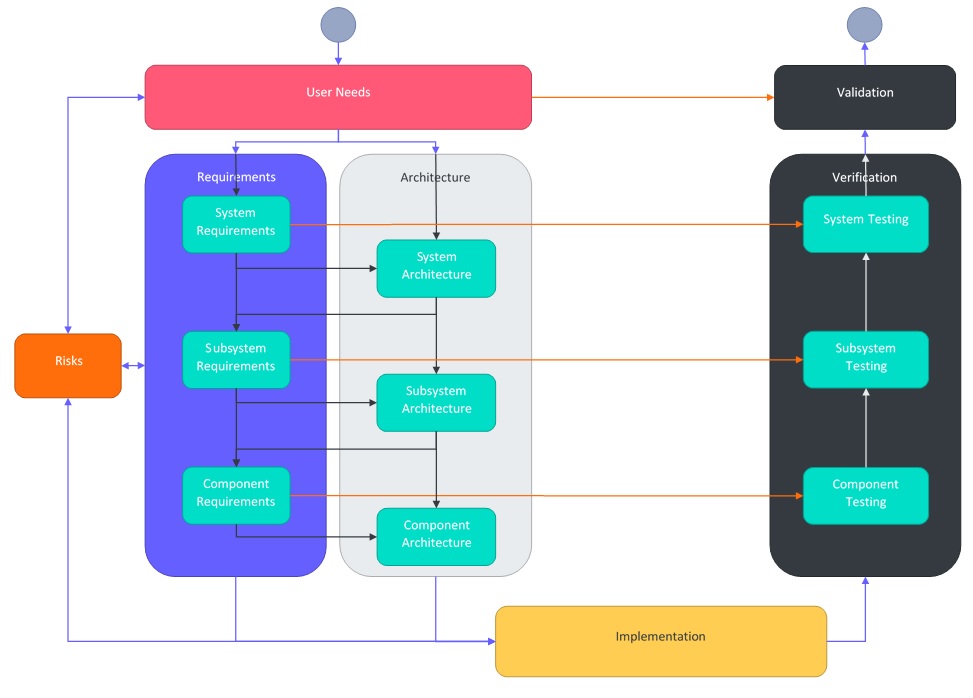
User Needs to Testing: A Complete Example – Part 1: Documentation Overview
This is the first part in a series which steps through how to create design and development documentation from user needs to test cases.
This part covers an introduction to the documentation hierarchy and how documents interact with each other.
Quick Tips: Avoid Delays with the Notified Body
Waiting until the last minute to notify your Notified Body about bringing new medical devices to market or about changes to a medical device on the market can result in delays.
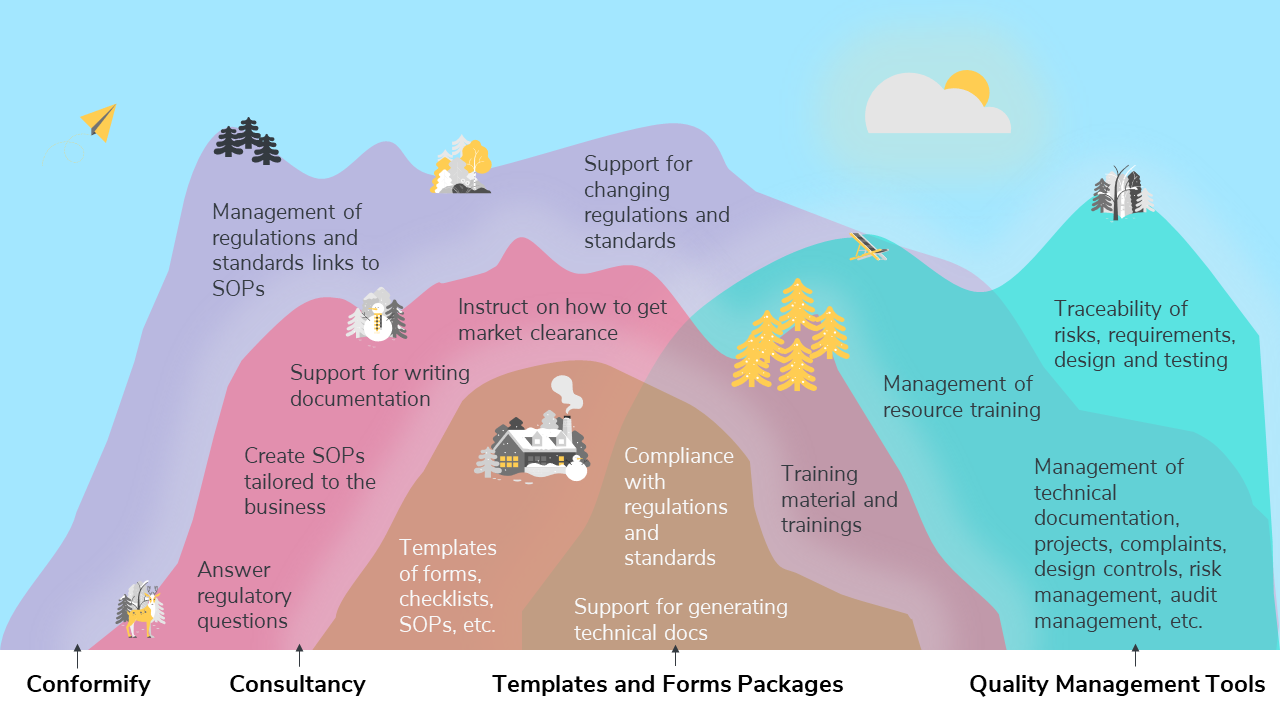
Venn diagram comparison of different quality management services and products
This Venn diagram compares Conformify’s products and services offering to other products and services such as consultancy, templates and forms packages, and quality management tools.
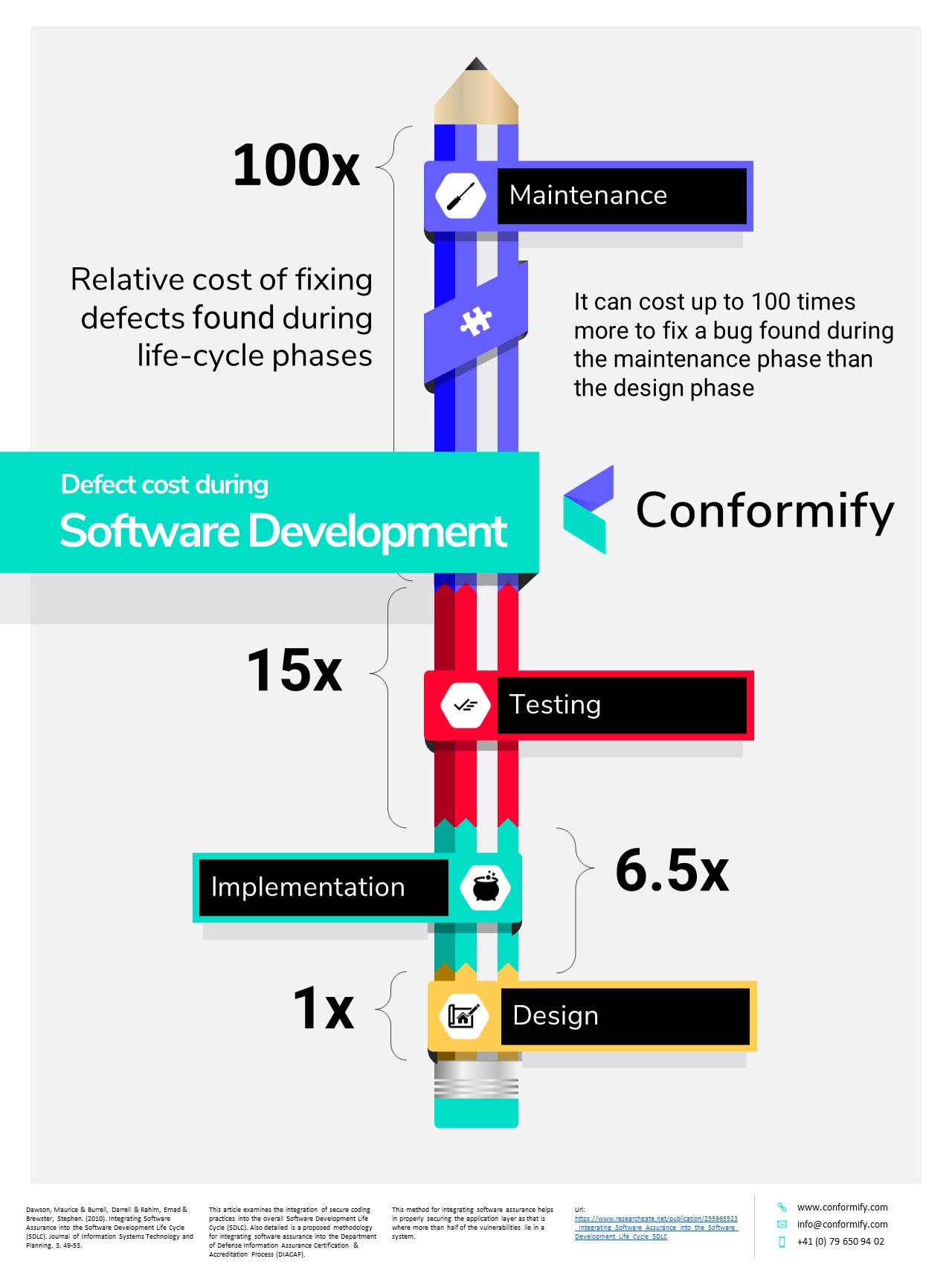
Relative cost of finding defects during the Software Life-cycle
Concentrating your efforts early in development of software can greatly reduce your overall costs. A study by IBM in 2010 found that as each life-cycle phase progressed, the cost of finding bugs increased exponentially.

Quick Tips: Comply to Standards
A quick tip on how to incorporate compliance to standards as part of your design and development process.
5 steps to make Conformify work for you
Find out how Conformify shows you exactly what you need to do to bring a device to market while ensuring you are compliant.
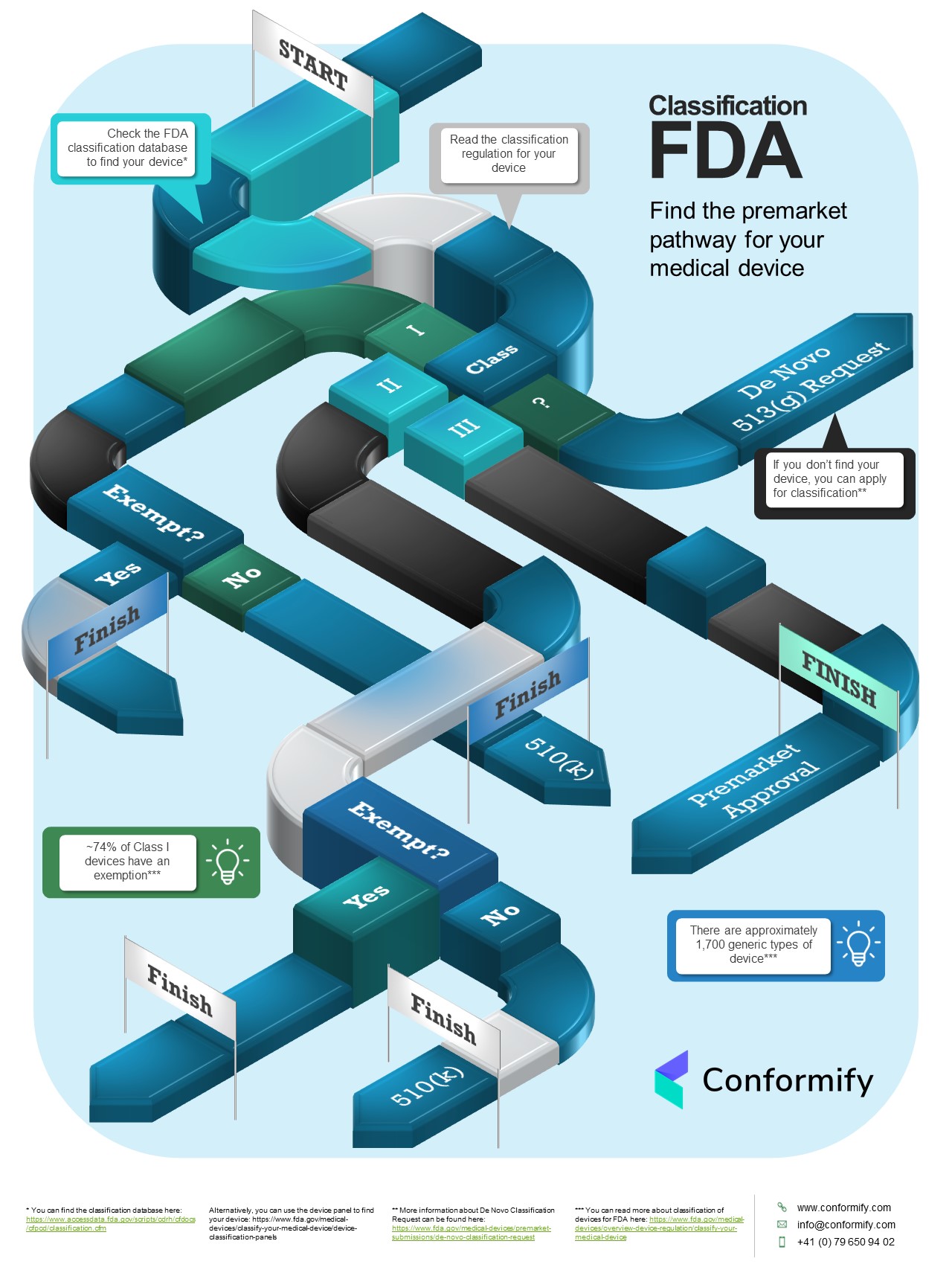
FDA Classification: Find the premarket pathway for your medical device
If you want to get your medical device to market in the U.S. you need to know its classification. To do this you need to:


