
Make a killer 510(k) – Part 2
One option to get more information about what the FDA may be looking for is to check the 510k database for similar devices that have been subjected to a Freedom of Information request, or FOIA Request.




One option to get more information about what the FDA may be looking for is to check the 510k database for similar devices that have been subjected to a Freedom of Information request, or FOIA Request.

Looking at the classification order or the reclassification order for device types can give you a lot of insight into the FDA’s thoughts on the benefit risk argument for your device. Addressing the FDA identified risks using the special controls identified for each risk correctly can boost your 510k clearance chances.
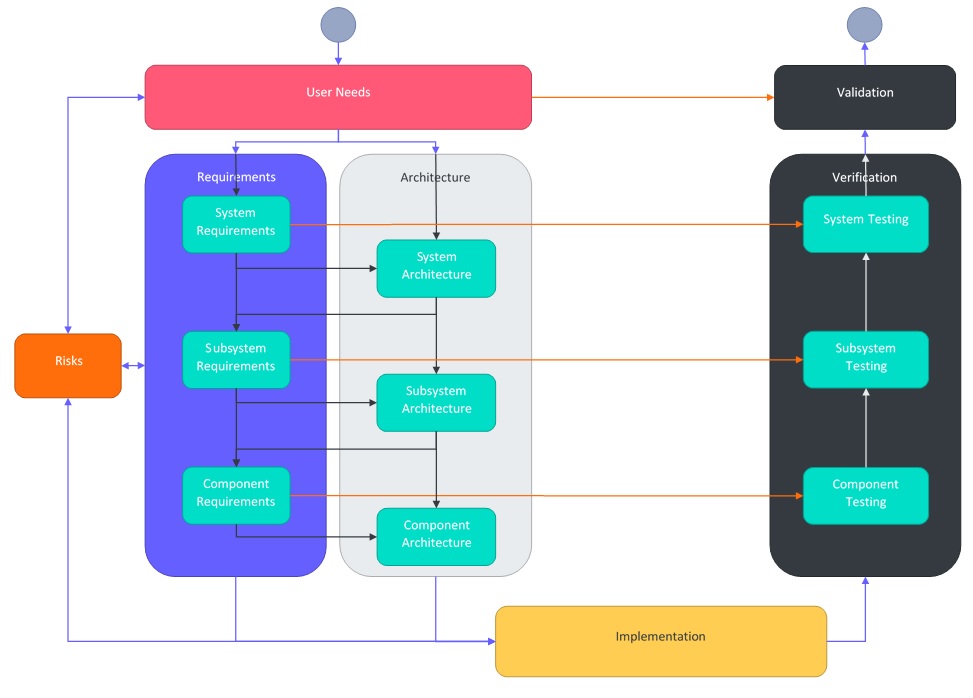
This is the first part in a series which steps through how to create design and development documentation from user needs to test cases.
This part covers an introduction to the documentation hierarchy and how documents interact with each other.
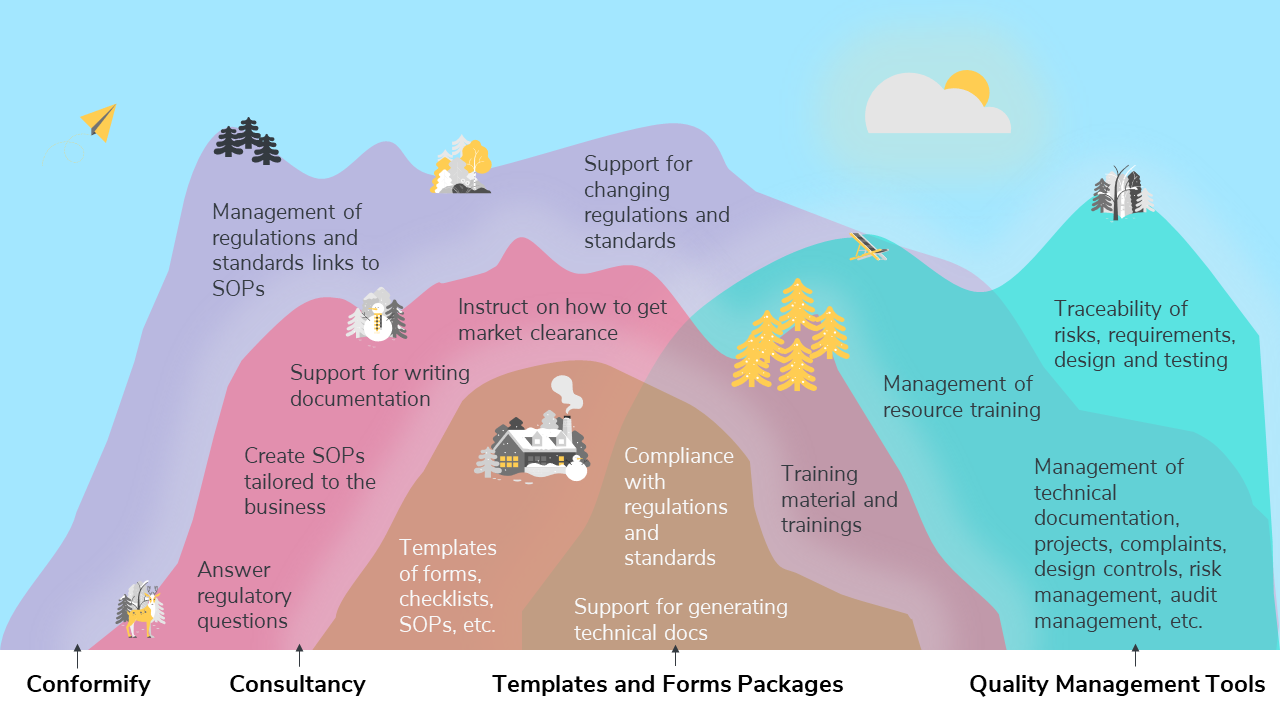
This Venn diagram compares Conformify’s products and services offering to other products and services such as consultancy, templates and forms packages, and quality management tools.

A quick tip on how to incorporate compliance to standards as part of your design and development process.
Find out how Conformify shows you exactly what you need to do to bring a device to market while ensuring you are compliant.
Think the waterfall methodology for design and development is the only compliant way to develop a medical device? Think again!
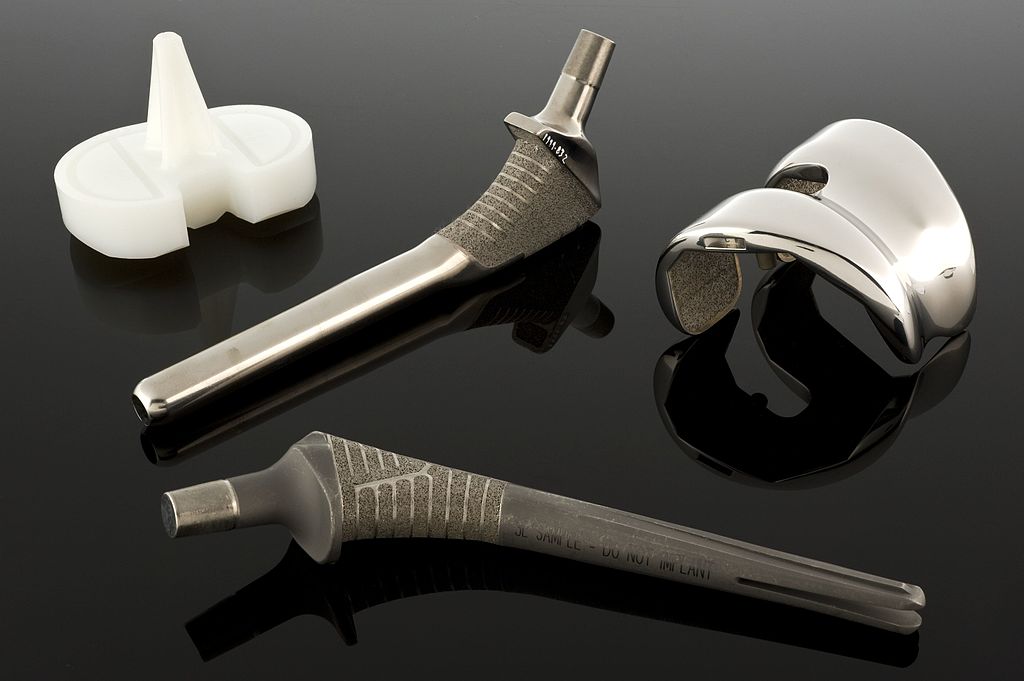
If you’re heading to the U.S. market with your medical device and can use the 510(k) program, you are going to need a predicate device

After years of development, hundreds of liters of coffee, numerous late nights and copious master and PhD theses later, you finally have a medical device
