
Make a killer 510(k) – Part 2
One option to get more information about what the FDA may be looking for is to check the 510k database for similar devices that have been subjected to a Freedom of Information request, or FOIA Request.




One option to get more information about what the FDA may be looking for is to check the 510k database for similar devices that have been subjected to a Freedom of Information request, or FOIA Request.

Looking at the classification order or the reclassification order for device types can give you a lot of insight into the FDA’s thoughts on the benefit risk argument for your device. Addressing the FDA identified risks using the special controls identified for each risk correctly can boost your 510k clearance chances.
We’ve all seen the tree swing analogy. Each member of a project has a different idea about the final product and it results in several misunderstandings.
How could this have been avoided? With good requirements!
Requirements are a contract between the business, the user and the design team. It transforms the user and business needs into a language that guides design, but does not dictate it. It captures all the goals that the design needs to meet in easy to understand language.

Documents in a medical device file may require duplicate information. For example, the intended use is a statement that appears in many documents such as the device description, project plan, risk management plan, or sterilization validation, etc. Modularizing the documents may improve your documentation quality by avoiding copy paste errors, but may also reduce the time needed to copy and paste as well as review burden.

Need to check the content of a standard before purchasing, so that you know whether or not it applies to your product? Or perhaps you want to clarify a reference to a standard in a document sent to you by one of your suppliers? You can access the many standards for free!
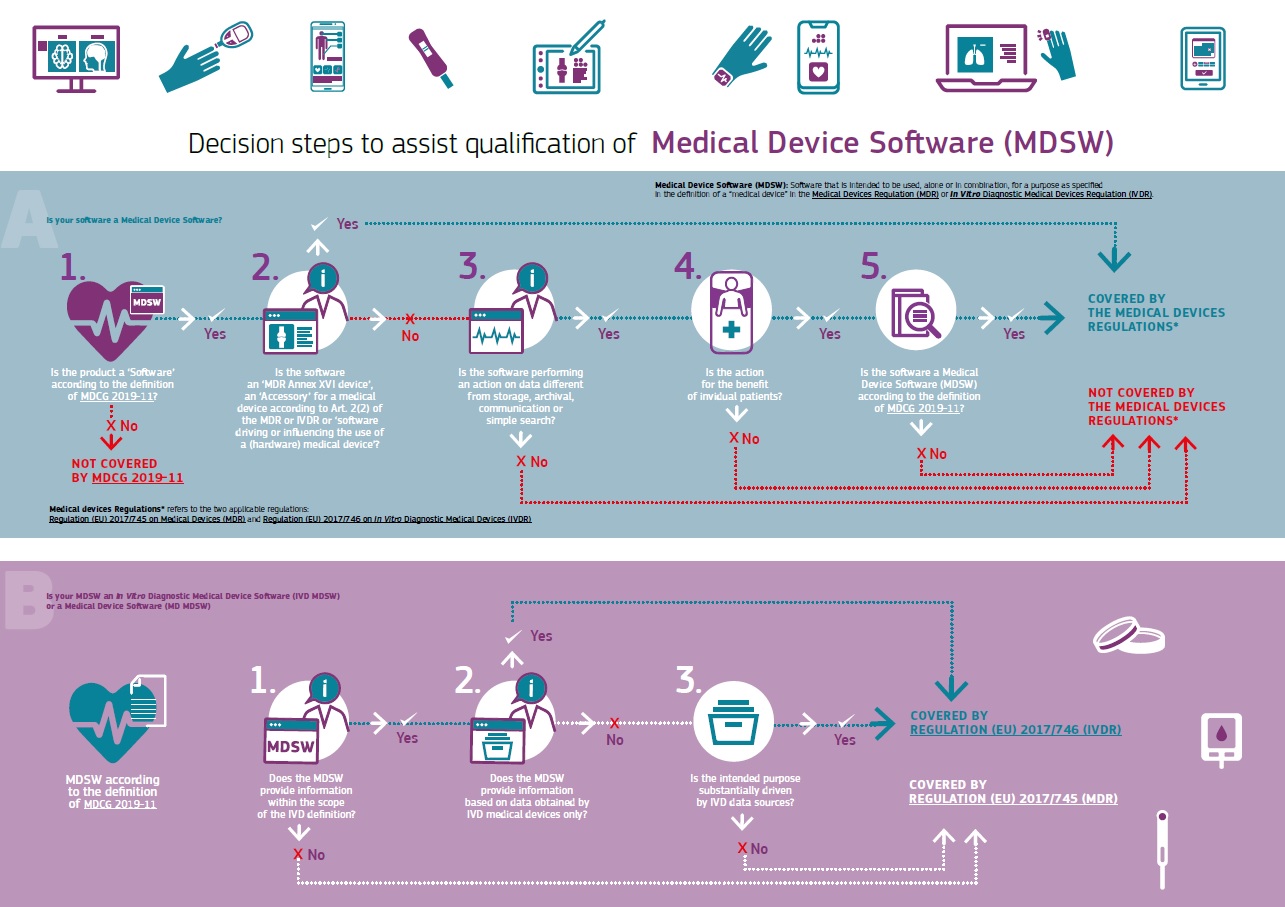
Is the software you’re developing a medical device?
This infographic published by the European Commission may help you figure it out.
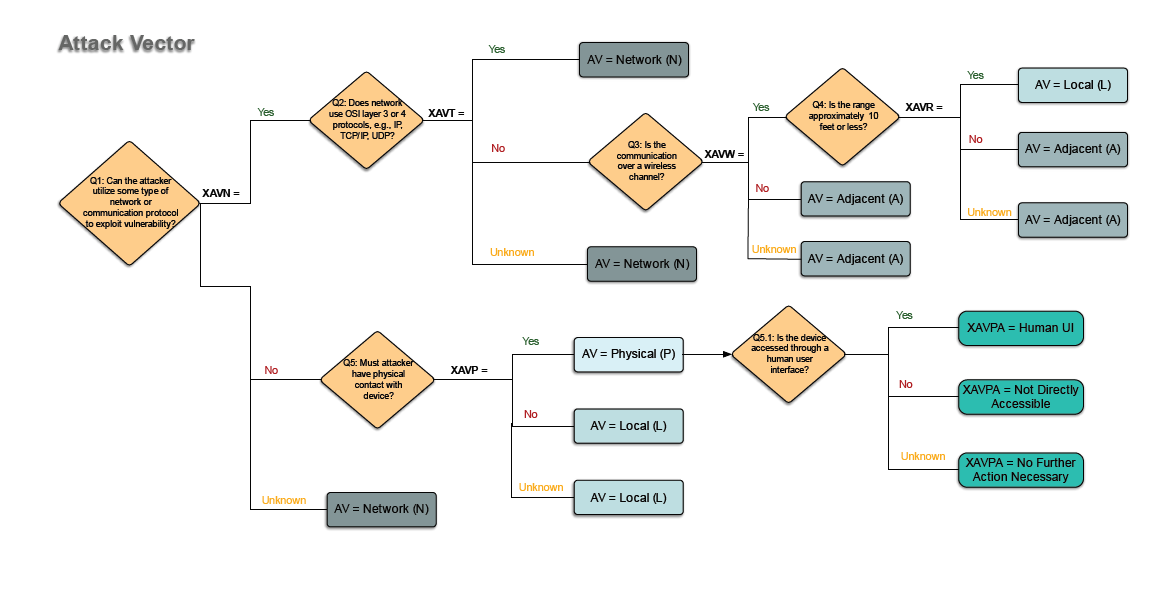
Cybersecurity is a growing concern to patients and manufacturers of medical devices. If you are medical device manufacturer with cybersecurity concerns and want to make your medical device more secure, you should consider cybersecurity as part of your risk management.
If you don’t already have an approach that works for you, consider using the ‘RUBRIC FOR APPLYING CVSS [Common Vulnerability Scoring System] TO MEDICAL DEVICES’ published by the MITRE Corporation.

Having trouble estimating risks in your risk management (according to ISO 14971)? Check out a few tips we have put together that may make things easier for you.

Here are 5 great tips on how to label the workplace to avoid quality issues and errors.
So, what are ‘User and Business Needs’ and why do we need them?
Medical devices, at a fundamental level, need to be safe and effective. More specifically, they need to be safe and effective when used in the context of their intended use. Regulatory markets around the world have recognized that ensuring that manufacturers consider users throughout design and development is critical to the success of a device and the safety of patients.
We take you through how to create a high quality user needs document with some examples.
