
ISO versus EN ISO
Ever wondered whether it was sufficient to use ISO 14971 instead of EN ISO 14971 for the European medical device market? This gives a quick breakdown of what the difference is, and why you need the EN ISO.




Ever wondered whether it was sufficient to use ISO 14971 instead of EN ISO 14971 for the European medical device market? This gives a quick breakdown of what the difference is, and why you need the EN ISO.
Find out how Conformify shows you exactly what you need to do to bring a device to market while ensuring you are compliant.
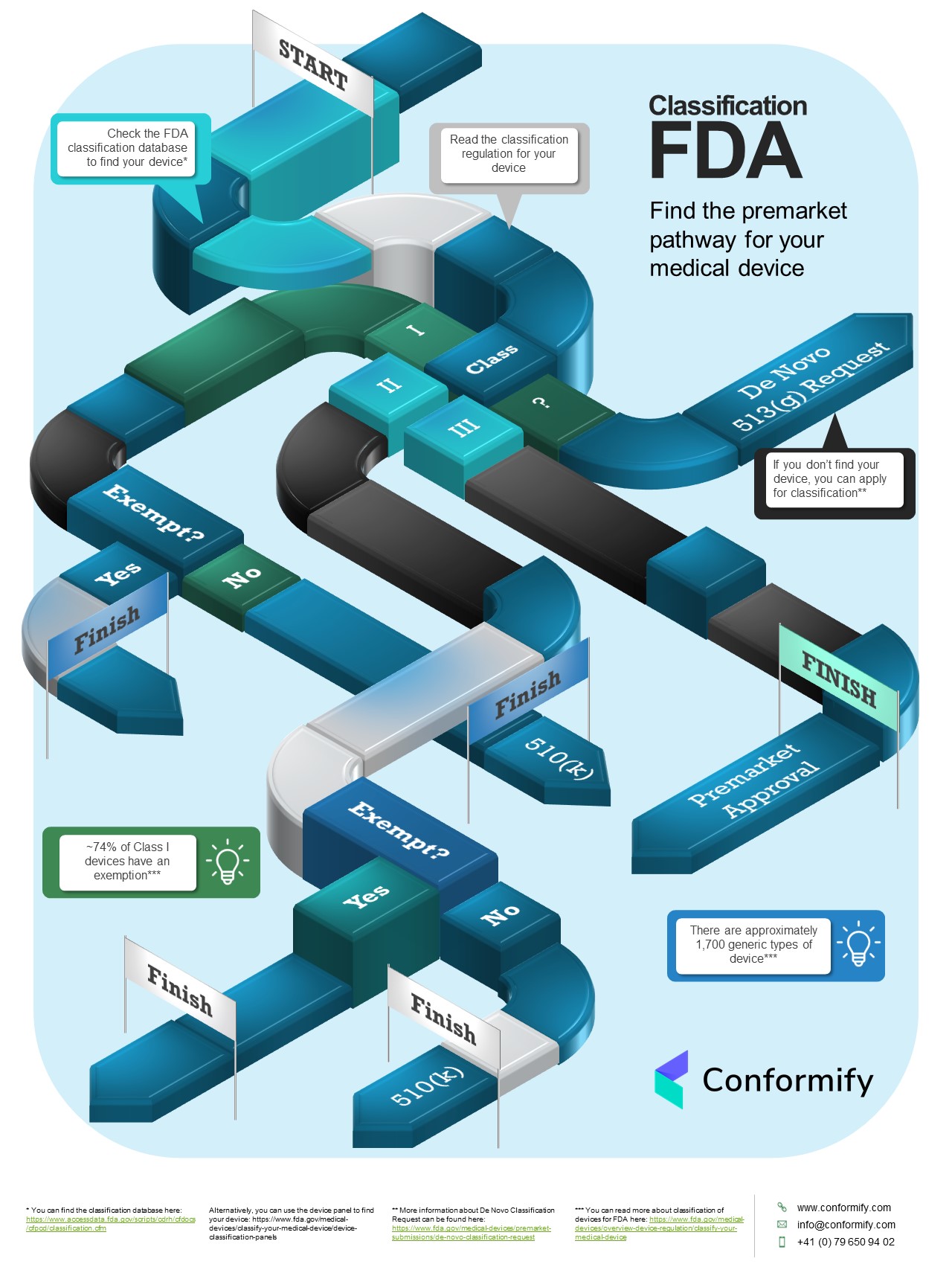
If you want to get your medical device to market in the U.S. you need to know its classification. To do this you need to:
One problem many quality management systems have, is being too rigid. If a procedure keeps ‘getting in the way’ maybe it is time for another approach.
