
Freedom of Information Act (FOIA) Requests
One option to get more information about what the FDA may be looking for is to check the 510k database for similar devices that have been subjected to a Freedom of Information Act request.
To do this, go to the 510k database: 510(k) Premarket Notification (fda.gov) and then select the checkbox ‘Redacted FOIA 510(k)’.
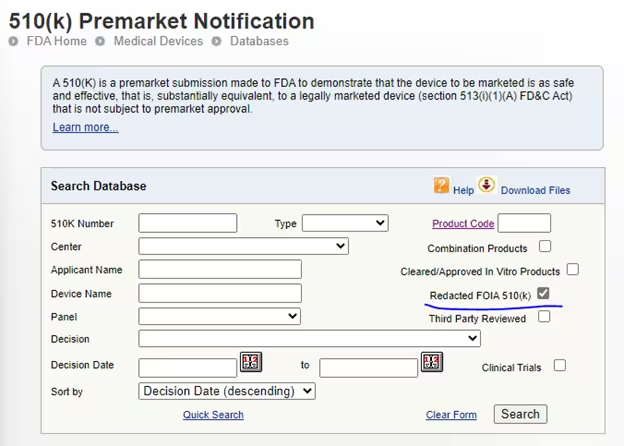
There are not typically a lot of these, so you may not find any for your device type. However, it can sometimes be useful to expand out your search to devices that are not exactly the same, but have some similar characteristics, because sometimes you can find some chunks of the FDA decision form which can give you insight into the FDA decision process.
Sometimes, you can get lucky and even get a chance to review the competitor’s User Manual, as in the example below:
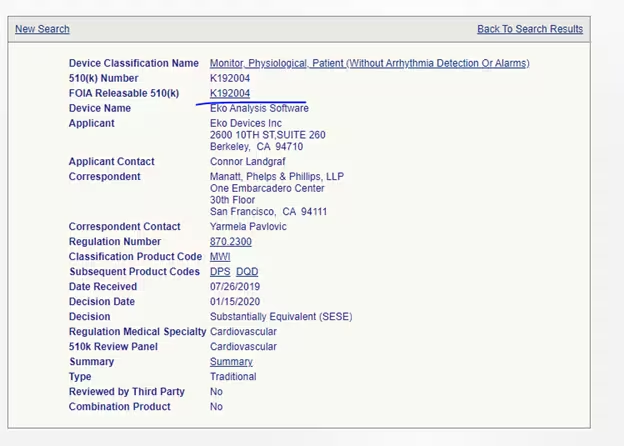
Just a note here: you can make your own FOIA requests. However, these cost money as the administration required to redact such large submissions can be tricky. In addition, the broader your request, the longer and more expensive it is going to be to address. You will certainly have more luck if you try to restrict your request to the FDA decision process rather than intellectual property of your competitor (which you are not going to be able to obtain through these requests).
The FDA currently states:
*Please note that requests for 510K, PMA, and De novo records are complex requests and take approximately 18-24 months to process.
So, if you are thinking of making your 510k submission anytime soon, it may not be worth the effort.
You can find information on the process here: How to Make a FOIA Request | FDA
You can find the online application form here: FDA Freedom of Information Act (FOIA) .
You can find information on the fees here: FOIA Fees | FDA
Need help?
Need help with your 510k? Conformify can help.
Just drop us a line to see what we can do for you!


