
Review the De Novo or Review the Federal Register
When a device gets classified as either class I or II, it typically happens through the De Novo process (De Novo Classification Request | FDA). You can find the searchable database of De Novos here: Device Classification Under Section 513(f)(2)(De Novo) (fda.gov).
Alternatively, it might go through the reclassification pathway. You can see the devices that have undergone reclassification here: Reclassification | FDA.
As part of these processes, the FDA will publish their reasoning for their decision as a classification order (published with the De Novo and in the Federal Register) or a reclassification order (published in the Federal Register). This reasoning also typically includes the list of risks that the FDA have identified, and the list of mitigations measures which then become the set of special controls.
Let’s get into an example of how you can make a submission better by taking a look at the recent De Novo classification order of the ‘Hearing Aid Feature (HAF)’ of Apple (DEN230081-S001.Letter.DENG.pdf (fda.gov)).
If you were to just look at the special controls for this device, you would see this:

This doesn’t provide you with a lot of insight into exactly what the FDA would like to see with the data. However, if you go to the classification order, you will see this:
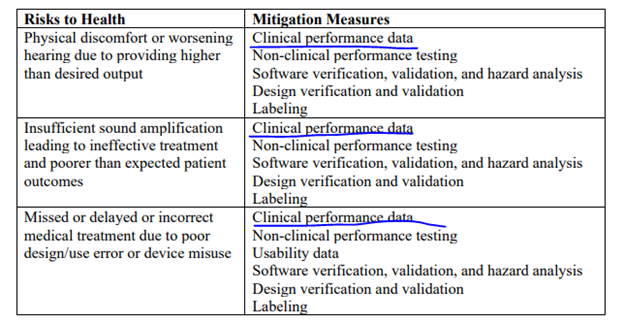
As shown above, clinical performance data should also be used to show that the device does not cause or effectively mitigates the risks to health the FDA have identified above. While it may not need to be the primary endpoint of your performance data testing, you will likely have to think about how you want to use the data to address these risks.
Need help?
Need help with your 510k? Conformify can help.
Just drop us a line to see what we can do for you!


