If you’re heading to the U.S. market with your medical device and can use the 510(k) program, you are going to need a predicate device and possibly reference devices. These are (more or less) devices that have already been cleared for market in the U.S. (except for those who were subject to the Pre-Market Approval program) that you can identity to determine substantial equivalence.
Although the FDA has a database in which you can find these devices, the summary documents contained there are very often very lean on details and you might find it difficult to use this information as your primary source. In addition, the database is expansive and the name with which companies market devices are sometimes not the name of the device under which they have submitted their 510(k) .
Step 1: Classify
The FDA suggest going to their Product Classification database and using that to search. You can start there and see how you go, but I find that it can be confusing if your device might fall into several categories. Instead, I recommend going directly to the regulations themselves and getting an overview of their chapter structure. When opening the chapter, sometimes it can become obvious whether or not your device would be considered to be classified under this aspect or not.
My suggestion instead is to head to the electronic Code of Federal Regulations parts 800-1299 and scroll down to sections 862 through to 898.
Each part here is divided into the type of device. Drill down these parts to find out how devices have been classified and then read more about the definitions of these devices according to the FDA. Then, make a list of the possible regulation numbers that your device falls into.
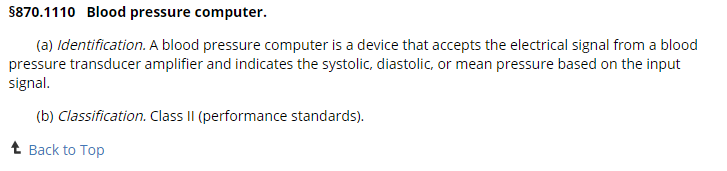
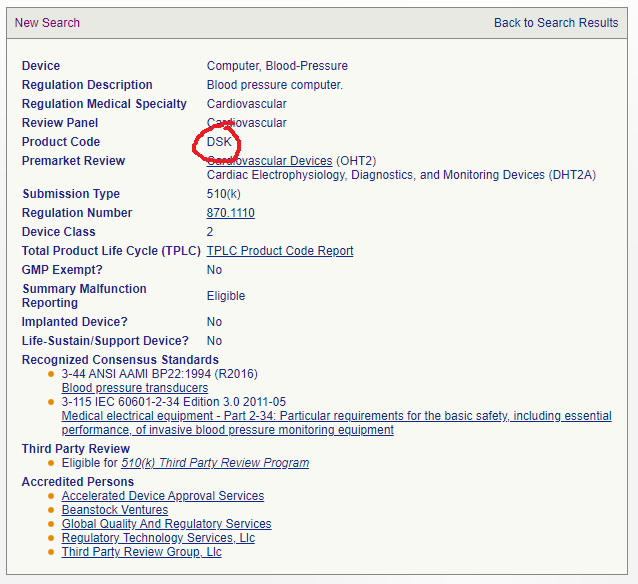
Once you have the regulation number, head back to the FDA Product Classification database and use these part codes and type these one at a time into the field named ‘Regulation Number’.
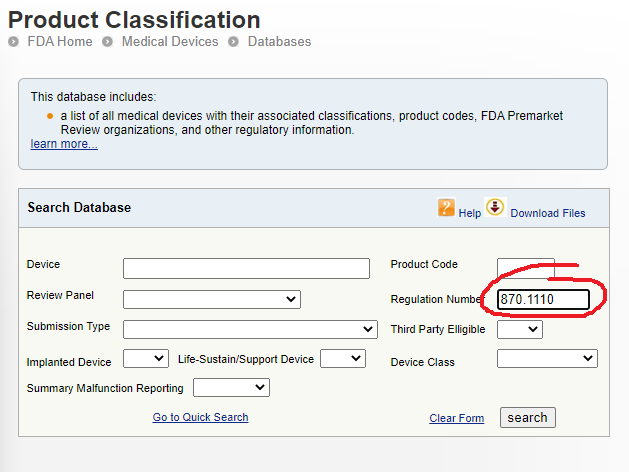
This will bring up some summary information about the regulation including the Product Code and even some important information about consensus standards of which you should be aware. Make a list of any product codes that may be of interest.
Step 2: Search for Contenders
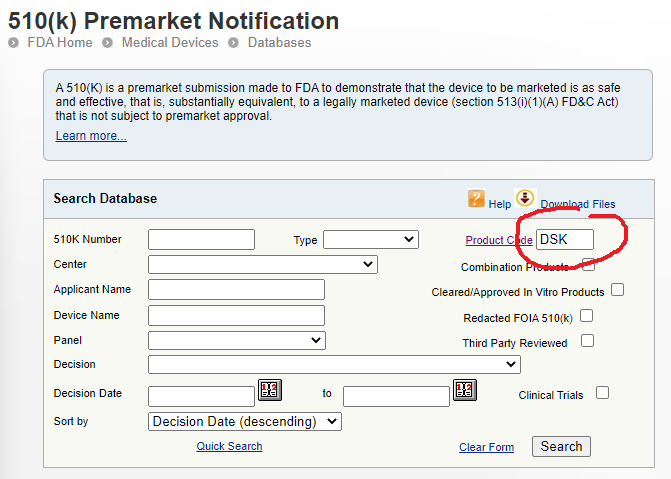
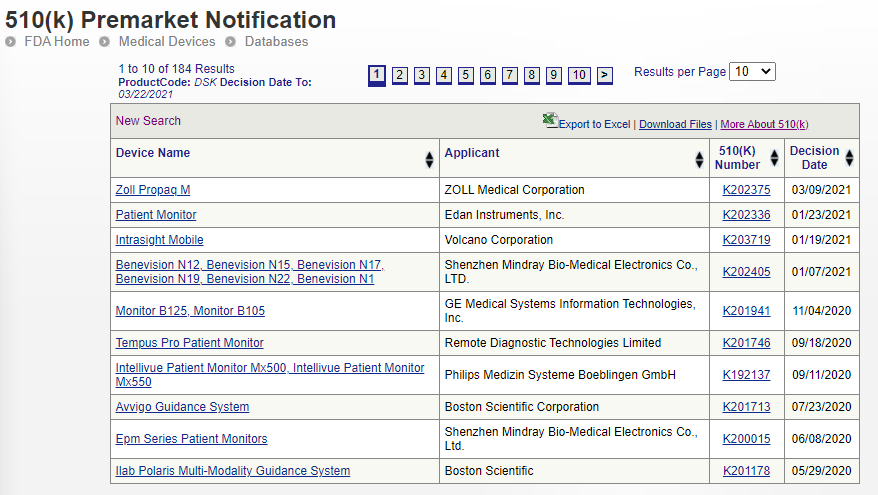
Once you have some promising product codes, you can head over to the Premarket Notification database and type these one at a time in the Product Code field. This will bring up a list of devices that have submitted successful 510(k) devices and therefore can be used as legitimate predicate or reference devices. Read each 510(k) summary (available by clicking ‘summary’), go to the respective company’s website, find out as much information you can about the device and then make a shortlist of potential contenders and their 510(k) numbers.
If you are not sure exactly what you are looking for in a predicate device, it might be helpful to check out this helpful guide about how substantial equivalence should be determined. The flowchart (at the time of writing it is Appendix A on page 27) gives a good overview of how aspects of the predicate device are compared against your subject device in order to make that determination.
Step 3: Get the Instructions for Use
(If you can…)
Now you have a shortlist, you need to go into more detail. The 510(k) can give you a good starting point as it usually provides the intended use and/or indications for use already. The wording of these can be tricky. If you see a difference between your intended use and theirs but you think they may only be ‘cosmetic’, you will probably need to confirm this by looking harder into their documentation.
The best documentation for this is their Instructions for Use. They are of particular interest because here you can also find hints about the kind of technology used, the kind of risks that are addressed, and the claims that the device makes to which you will need to show substantial equivalency. However, IFUs can be difficult to get a hold of. Sometimes, you might be lucky enough to find copies on the internet (and sometimes in other languages), but this is not always the case. If you are lucky enough to have contacts in the healthcare industry that work for hospitals or other healthcare institutions, it is possible that they may have access to online databases where many electronic copies of various devices preside. Or, they may even be able to get a copy from a device to which they have access.
If you really can’t get the instructions for use, sometimes you might find sufficient information contained in the scientific data to be able to paint a good enough picture of the context of use, risks and technologies used.
Step 4: Get the Scientific Data
Many devices spend time going through the research stage, which means that somewhere buried deep in scientific publications is likely to be some information about your predicate device. The difficult part is finding it. In early stages of device development, it is possible that the current manufacturer did not exist and instead a research institute was developing the device under another name. This makes tracking back from a 510(k) predicate device to its origins quite complicated.
To find the relevant scientific data, I suggest the following:
- Get a hold of one of the researchers that used to work on your own device. A PhD student or Master’s student are of particular interest because they will have written a thesis on the subject and should be very familiar with the device landscape. This will most probably know the most important list of research institutes which work on the subject and may even be able to recognize your predicate device. This will help narrow down your search at least to certain research institutes and their partnerships with the company that builds your predicate.
- Use scientific literature search libraries to get the most comprehensive results. If you are lucky enough to have access to PubMed, or contacts that do, this is a great place to start. If not, you can try places like Google Scholar but the reality is, you really need access to the scientific papers in the results to be able to correctly carry out your job of determining substantial equivalency with your predicate.
- Check out any clinical trials listed for your device. Even though it is possible that your device has not published their results (even though they really should!), you might still be able to find out the wording used in the clinical trial and then be able to trace this back to any academic papers written about the results. Of particular interest is also the method that they used which can sometimes also describe the technologies and context of use of your predicate device. It may also give you hints as to which research institute with whom they may have partnered and therefore find other linked academic papers written by them.
- Talk to people that go to medical device fairs. Sometimes you can get a lot of information about a device simply by being able to see it in real life. Other times, it can be useful to get every single piece of marketing material that you can get your hands on.
Step 5: Check Post-Market Safety Data
Finally, if your predicate device or devices within your classification group have reported any adverse events and/or performed any recalls, it is important to know that now. First (and most important) of all, you must make sure that your device has addressed any safety or effectiveness issues that may have been raised in adverse events reports from similar devices. This is of course paramount to the safety of patients. And second of all, where trends have appeared or where poor clinical outcomes have been identified with certain technological aspects, the FDA may pay particular attention to your scientific argumentation for how you have addressed these issues. This may mean providing more data or more testing on the subject than your predicate device.
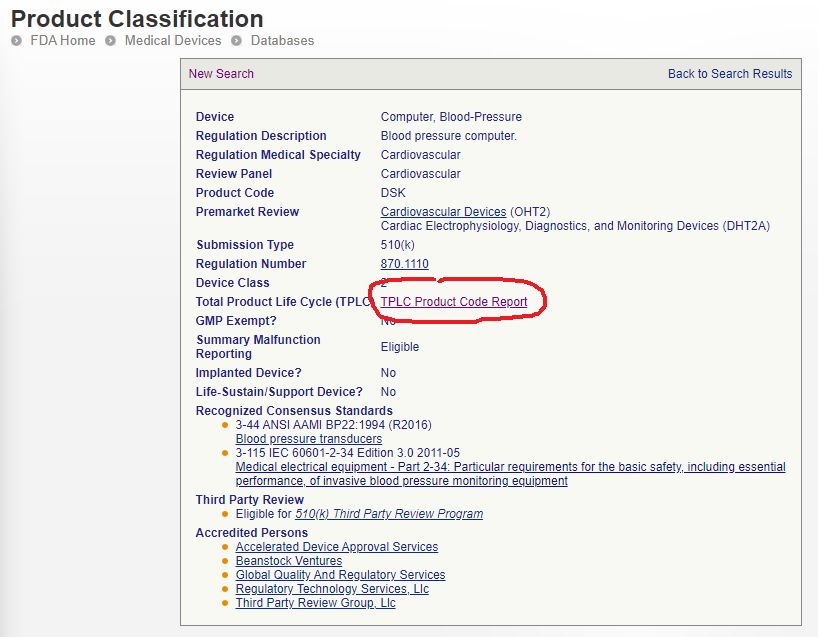
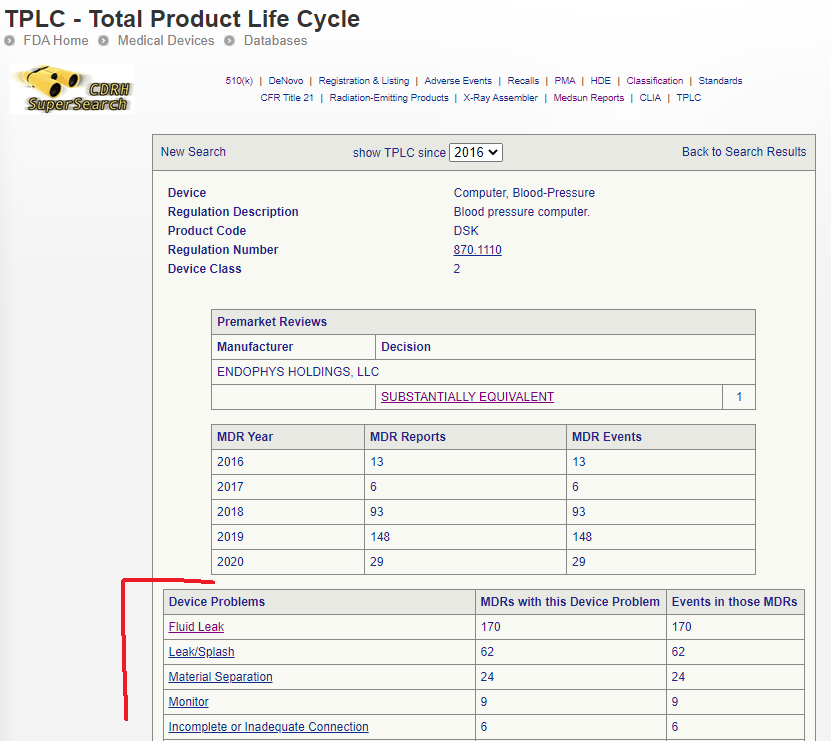
To make a quick check, go to the Product Classification database and type in the regulation number again. Then click on the ‘Total Product Life Cycle’ link to get more information.
We can also help you find your predicate device(s), reference device(s) and relevant literature if you need. Check out our Micro-services, or head directly to our Predicate Search product.