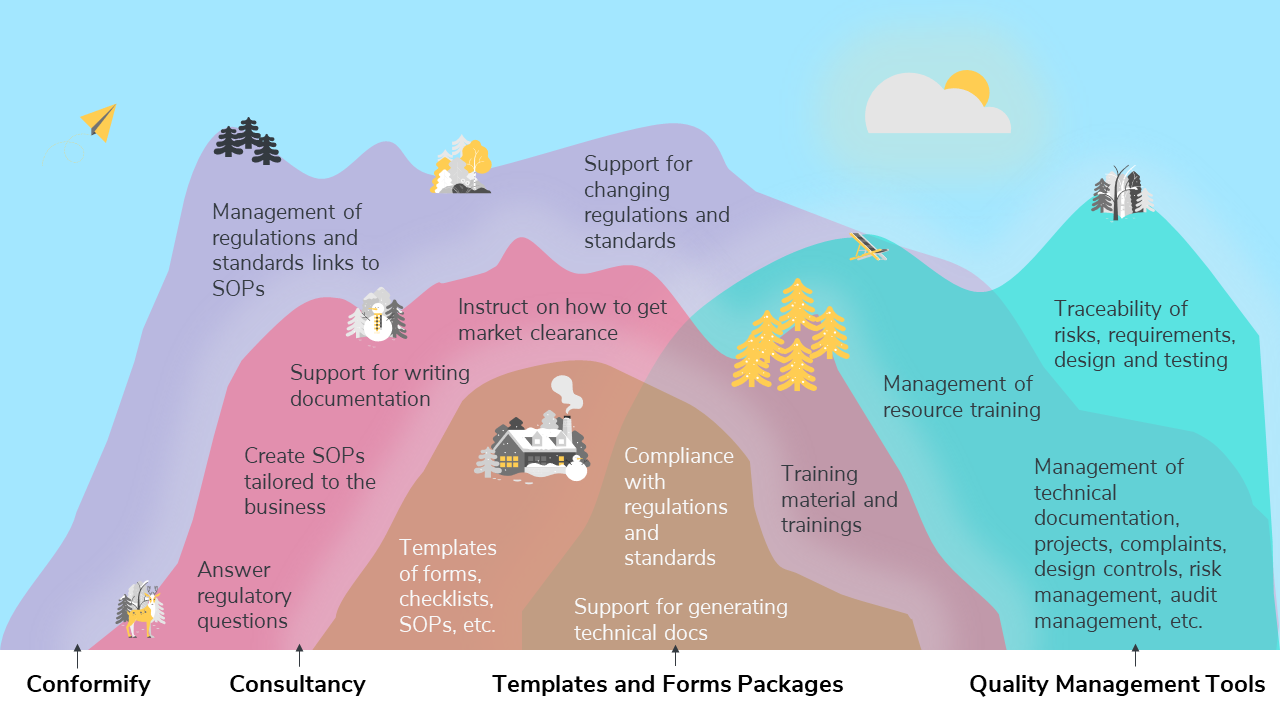
Comparison of different quality management services and products
| Templates and forms packages | Consultancy | Quality management tools | Conformify | |
|---|---|---|---|---|
| Support for generating technical docs | X | X | X | X |
| Compliance with regulations and standards | X | X | X | X |
| Templates of forms, checklists, SOPs, etc. | X | X | X | |
| Training material and trainings | X | X | X | |
| Instruct on how to get market clearance | X | X | ||
| Support for writing documentation | X | X | ||
| Create SOPs tailored to the business | X | X | ||
| Answer regulatory questions | X | X | ||
| Management of technical documentation, projects, complaints, design controls, risk management, audit management, etc. | X | X | ||
| Management of resource training | X | X | ||
| Traceability of risks, requirements, design and testing | X | |||
| Support for changing regulations and standards | X | |||
| Management of regulations and standards links to SOPs | X |
Support for generating technical docs
We include descriptive text about what sort of content should be created and where and even may provide example text for convenience.
Compliance with regulations and standards
We ensure that our provided documents adhere to the most important standards and regulations that for the U.S. and E.U. market. We can also tailor these documents for you to meet other regulations or standards where necessary.
Templates of forms, checklists, SOPs, etc.
We provide the necessary templates, forms, checklists, etc. to complete your quality management system.
Training material and trainings
We provide training material and trainings not only on how to use our software, but also for our SOPs.
Instruct on how to get market clearance
Our SOPs provide the step-by-step instructions on how to bring your device to market in the U.S. or E.U. so you can do it as easily and efficiently as possible.
Support for writing documentation
We can also help you write your documentation if you need more support.
Create SOPs tailored to the business
Our SOPs are designed to be as modular as possible so that most business simply need to select the kind of device they are building and receive all the building blocks necessary to have a complete, compliant and efficient quality management system. However, we are also happy also tailor your SOPs for you so that your quality management system can meet your exact needs.
Answer regulatory questions
Got a regulatory questions? We can help you get an answer.
Management of technical documentation, projects, complaints, design controls, risk management, audit management, etc.
Our software provides an easy and efficient method of helping you produce and manage all your documentation. The software maintains all your files (technical files, design history files, device master files, device master records, risk management files, complaint files, CAPA files, and many more!) in an easy to use format.
Management of resource training
Need to track which of your employees are trained on which SOPs? Conformify does that for you too.
Support for changing regulations and standards
As our SOPs are linked to the relevant standards and regulations, we can easily let you know if updates to these standards or regulations affect your quality management system and can provide you with updated SOPs so that you are always compliant.
Management of regulations and standards links to SOPs
If you need to check whether your quality management system has full coverage, you can check which regulations are linked to which SOPs. This is also useful for audits as you can quickly present how your QMS meets your regulatory obligations.
If you want to find out more about Conformify’s offering, check out the following links:
https://conformify.com/software/
https://conformify.com/micro-services/


